By Daniel Drachman, MD, as told to Stephanie Watson
Myasthenia gravis (MG) has been treatable for nearly 100 years. In 1934, the Scottish doctor Mary Broadfoot Walker, MD, discovered that the drug physostigmine improved muscle function in people with the disease.
In myasthenia gravis, abnormal antibodies reduce the number of receptors for acetylcholine on muscle cells. You need acetylcholine receptors for your muscles to function normally. The antibodies interfere with the sending of the chemical signal from the nerve to the muscle that makes the muscle contract.
Today, pyridostigmine (Mestinon), a drug that’s a relative of physostigmine, is used to slow the breakdown of acetylcholine. It helps with the symptoms of MG, although it doesn’t treat the underlying autoimmune problem. It’s a sort of like a bandage.
We also have drugs that suppress the immune system, including the steroid prednisone, azathioprine (Imuran), cyclosporine (Sandimmune, Neoral), mycophenylate mofetil (CellCept), and tacrolimus (Prograf). These drugs effectively counteract the autoimmune problem. But they need close watching and careful management by a doctor because of their side effects.
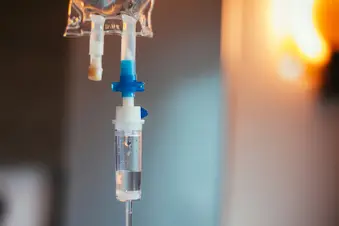
Plasmapheresis, also called plasma exchange, is used for people who are seriously ill and haven’t responded to other treatments. During this procedure, the fluid part of the blood (plasma) that contains the abnormal antibodies is removed and replaced. More recently, we’ve used intravenous immunoglobulin (IVIg), which is an infusion of blood antibodies purified from donors.
Thymectomy
Thymectomy is surgery to remove the thymus gland. It has been around for a long time, but only in 2016 did a thorough study confirm that it works for myasthenia gravis.
The thymus is an important part of the immune system, and it’s abnormal in about 75% of people with myasthenia gravis. By removing the thymus, you remove one of the main sources of autoimmunity that causes myasthenia gravis. After thymectomy surgery, many patients gradually improve. Thymectomy is the only treatment that sometimes leads to a cure.
Monoclonal Antibodies
These newer medications target specific parts of the immune system. Eculizumab (Soliris) blocks the part of the immune system called the terminal complement cascade, which binds to antibodies and damages the junction between the nerve and muscle (neuromuscular junction). Sometimes the effects of this drug are dramatic.
Rituximab (Rituxan) is another monoclonal antibody. It lowers levels of B cells, immune cells that produce antibodies. Rituxan isn’t FDA-approved for myasthenia gravis, although it is approved for lymphoma.
Belimumab is another new monoclonal antibody on the horizon. It blocks something called B-cell activating factor and may suppress antibody production.
New Class of Medicine
Another new drug is called efgartigimod. It leads to the rapid removal of antibodies, including the autoantibodies that cause myasthenia gravis. It has an effect similar to plasma exchange, but it uses an antibody to remove the autoantibodies that your body has made to attack the acetylcholine receptor. In fact, I’ve called this drug “plasma exchange in a bottle.”
Efgartigimod has another benefit. Unlike Rituxan and other immunosuppressive treatments, it doesn’t damage the immune system cells that make antibodies. Patients who receive Rituxan don’t mount a good antibody response when they receive the COVID-19 vaccine. People who receive efgartigimod can be effectively vaccinated against COVID-19.
This drug also works faster than many current treatments. Imuran and CellCept typically take many months to work. Because of its effectiveness and speed, efgartigimod could turn out to be one of the best treatments for myasthenia gravis. It might even replace steroids.
Much Less Serious
We now have a large group of treatments for myasthenia gravis and more coming down the pipeline. Nearly everybody with this condition can now be well treated and managed.
One day, we might even be able to cure myasthenia gravis. A few years ago, I figured out a way to turn off the immune response to acetylcholine receptors specifically, without affecting any other part of the immune system.
We genetically engineered certain cells of the immune system, which we called “guided missiles.” When injected into the body, those cells targeted and killed the specific T cells that were involved in producing harmful antibodies. When we injected those cells into mice with myasthenia gravis, they essentially stopped the disease without reducing the overall immune response. This procedure is too complicated to use in humans yet, but the point is, our research proved that it can be done.
Photo Credit: iStock/Getty Images
SOURCES:
Daniel Drachman, MD, professor of neurology, neuroimmunology, and neuroscience, Johns Hopkins University, Baltimore.
Journal of the History of Neurosciences: “The contribution of Dr. Mary Walker towards myasthenia gravis and periodic paralysis whist working in poor law hospitals in London.”
FDA: “FDA Approves New Treatment for Myasthenia Gravis.”
